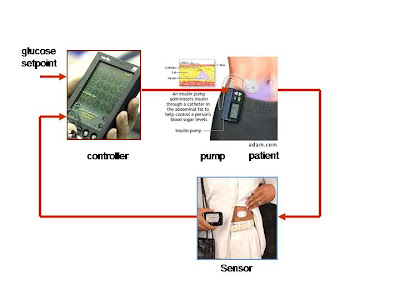
Completing the Loop
Monday, November 23, 2009
Costly or Not?
In most articles I've come across, there is no specific price which is given as an estimate for the device, but most seem hopeful for the price to be right around that of the CGM. Also, the JDRF is pushing towards attempting to push for insurance coverage of the device once it is approved for use by the public.
Also, speaking in terms of overall cost, this product would greatly reduce the amound patients would spend treating their Diabetes, it would be very cost-effective in the long term.
Here are a couple links which lightly discuss this topic:
http://artificialpancreasproject.com/faq/#approximately-how-much - this link also answers many questions which one may have about the device, it's development, etc..
http://web2100.jou.ufl.edu/index.php?id=1237
Sunday, November 22, 2009
Artificial Pancreas Just Years Away, Experts Agree
Maggie Fox July 26, 2008
This article focuses on the timeframe for when an artificial pancreas may come full-swing to market. The general consensus among researchers working on the project is that a fully-functioning artificial pancreas will be on the market within five years. Currently, many clinical trials have shown that the artificial pancreas is near perfect, with the new pancreas regulating glucose levels much more thoroughly than that in the diabetic patient’s body. As people with diabetes cannot constantly check their glucose levels, generally they are limited to checking at most twenty times a day. While the artificial pancreas is not perfect yet, it monitors glucose levels continuously and thus still may function better than the actual, bodily pancreas, particularly for young children who cannot check insulin levels themselves and thus require constant parent supervision. But before the new technology can be marketed, several of its quirks must first be fixed. Researchers are confident that these will be fixed in several years, but many diabetic patients are extremely anxious for this new technology to reach the market, having found immense success in clinical trials.
"Artificial Pancreas" For Some Diabetics
In Summer 2008, CBS News did a story on Julie Anne Ressler. She is a mom, a doctor, and a Type 1 diabetic. For the majority of her life, she has had to prick her finger more than 10 times a day in order to monitor her blood glucose levels and to determine how much insulin she should take. She says that she is constantly reminded throughout the day that she has diabetes because she has to check her numbers so much. Recently, in Los Angeles, she was put on a test trial with the artificial pancreas. The doctor who used her for the test trial said she loved it because she wasn't worried about her diabetes all day; the artificial pancreas took care of everything. Without any direction from herself, the machine was able to successfully detect blood glucose levels and then give the appropriate amount of insulin. Again, as mentioned in previous posts, everyone is anticipating the approval of the artificial pancreas by the FDA.
In order for biotechnology to be practiced effectively, the human costs and affects need to be weighed just as much as the actual medicine and science. This CBS show elucidates this point; it hones in on the human perspective of the artificial pancreas. Julie Anne Ressler explained how life was exceptionally better with the artificial pancreas and that she was lucky to have gotten the chance to try it for at least a few days.
With this in mind, I was upset that with positive patient feedback, the FDA still had not given the artificial pancreas approval. I decided to look at the FDA website for more information regarding the much anticipated artificial pancreas approval. In order to expedite the approval, the FDA has created the Interagency Artificial Pancreas Working Group (IAPWG). This group works with private organizations, patient groups, academic researchers, product developers, industry and other government groups in order to figure out ways to speed up research and development. Most of the problems that are interfering with approval involve technological difficulties ( ie imperfect algorithims and mismatches between blood and interstitial glucose levels)
Here is the CBS showing about Julie Anne Ressler and the artificial pancreas.
http://www.cbsnews.com/sections/i_video/main500251.shtml?id=4327391n
http://www.cbsnews.com/stories/2008/08/06/earlyshow/main4324074.shtml
http://www.fda.gov/ScienceResearch/SpecialTopics/CriticalPathInitiative/ArticlesandPresentations/ucm077537.htm
Medtronic begins international sales of semi-closed loop Paradigm Veo diabetes device
http://www.medcitynews.com/index.php/2009/09/medtronic-begins-international-sales-semi-closed-loop-paradigm-veo-diabetes-device/
Saturday, November 21, 2009
Researchers Outline Steps to Artificial Pancreas
In an article from Critical Endocrinology News, information is presented on the steps towards closing the loop in the Artificial Pancreas Project. The JDRF has been working to solve many issues which have been problematic to completing this project. These include adding an automated pump shut-off, having "hybrid" glucose cont
 rol, the possible inclusion of glucogen, silico modeling and incorporating an in-hospital closed loop system.
rol, the possible inclusion of glucogen, silico modeling and incorporating an in-hospital closed loop system.The automated pump shut-off will control the information sent from the sensor to shut off insulin delivery; this whole process will help to prevent hypo/hyper glycemic levels in patients.
"Hybrid" glucose control refers to a new attempt at regulating infusion rates. Dr. Stuart Weinzimer of the department of pediatrics at Yale University and his associates found that the controlling algorithm was leaving significant postprandial excursions with the system because of delays in insulin absorption. Weinzimer and his associates proposed introducing small manual priming doses prior to meals during close looped control. The results were not quite what the researchers expected, but they are optimistic about the feasibility of the closed loop control with the interaction of manual doses. “Further refinements are necessary to accelerate insulin action and decay rates to minimize postprandial glycemic excursions and reduce late postprandial hypoglycemia,” said Weinzimer.
Inclusion of glucogen is a debatable area, but according to Edward Damiano, Ph.D, of the division of Biomedical Engineering at Boston University, the introduction of glucogen in a truly closed loop system is neccessary. “I’m pretty convinced that you need a counterregulatory hormone. In real life, tremendous precipitous drops in blood sugar can happen due to changes in insulin sensitivity in a very short space of time. ... There’s no way a machine can prevent something like that, that quickly. Glucagon works extremely fast,” he said.
Another topic, in silico modeling refers to computer simulation of patient and device variables. Typically, there is a need to study animals before humans, but this is costly and time consuming. These computer simulations can rapidly assess the feasibility of various algorithms for human trials and therfore save years of time which would have been spent on testing.
Finally, in-hospital closed loop systems could be very beneficial for hospitalized patients. It would help to take strain off of nursing staffs who cannot manage to closely monitor patients continuously. And since most initial clinical trials have taken place in hospital settings, it would not be an uncommon practice.
All in all, these topics are among the final obstacles and decisions which must be solved and determined for the completion of the closed loop system. Just a few steps now stand between us and a functional Artificial Pancreas.
http://www.jdrf.org/files/General_Files/APP/2008/Clinicalendocrinologynews_AP.pdf
Sunday, November 15, 2009
“Latest Advance in the Treatment of Diabetes: An Artificial Pancreas”
After reading the November 2008 article (“Researchers Developing Artificial Pancreas to Treat Diabetes”), I wanted to find a more recent article which might shed some insight on new developments with the artificial pancreas. This article, published in July of 2009, focuses on the life of a 14-year-old girl, Sarah, who has briefly tried using an artificial pancreas and has experienced amazing results. According to the article, Sarah must test her blood sugar ten or more times a day, as well as count her carbohydrate intake and adjust the amount of insulin she gives herself based on this information. But for a few days Sarah didn’t have to worry about such tedious measures. Instead she was able to test out an experimental artificial pancreas, one of approximately 75 diabetes patients across the U.S. taking part in the clinical trials. Every minute, the artificial pancreas delivers the necessary amount of insulin to control sugar levels. As 7.8 percent of the U.S. population has diabetes, a device which could procure these amazing results would dramatically improve the lives of millions. Currently, some diabetic patients already have continuous sensors which monitor sugar levels in the body, as well as pumps that dispense insulin. The goal of the new artificial pancreas is to link these two components. With the new technology, a sensor beneath the skin sends a signal to the transmitter, which then goes to a control box. The control box in turn tells the pump how much insulin to release. The patient feels nothing while this occurs, and can eat whatever she wants while the artificial pancreas makes all of the adjustments. But the technology is far from perfect, as researchers are now trying to make the device smaller and more precise.
http://abcnews.go.com/Health/MedicineCuttingEdge/story?id=8043506&page=1
“Researchers Developing Artificial Pancreas to Treat Diabetes”
This article from Scientific American provides a basic overview of the current artificial pancreas technology, although it must be noted that the article was written several months ago and therefore the technology may have improved slightly since then. Patients with diabetes must constantly attempt to maintain the right blood sugar level, a difficult and time-consuming job. These patients make little or no insulin, a hormone which is normally produced in the pancreas and which breaks down food into energy. The problem with insulin injections is that they prevent too much sugar from accumulating in the blood, potentially leading to a diabetes-induced coma. Therefore scientists in England and the United States are working on an artificial, iPod-sized pancreas to be worn outside the body. The artificial pancreas consists of two main parts: a continuous glucose monitor and an insulin pump. A computer chip embedded in the insulin pump takes in information about the patient’s blood sugar levels from a glucose monitor which then attaches to the skin. The pump would be worn on a belt or in a pocket and would use the information to inject the necessary amount of insulin through a needle in the belly. This would allow the patient to have the right amount of insulin in her blood without having to manually change the dosage. The projected cost of the technology has not yet been determined, and Bruce Buckingham of Stanford University, where some of the research is occurring, guesses that the device is still five to ten years away from the market. Devices made by the companies Medtronic Diabetes, Abbott Laboratories, and Johnson & Johnson are testing the software. Although the technology is undoubtedly promising, in order to truly be effective it must be provided at a relatively low cost; otherwise, the millions of people who would benefit may not have access. And if universal healthcare is implemented, will the artificial pancreas be provided for? How will the accessibility differ between different age groups? These are the types of questions which must be addressed in order to determine the feasibility of this new technology.
http://www.scientificamerican.com/blog/post.cfm?id=researchers-developing-artificial-p-2008-11-03
An Artificial Pancreas
http://www.technologyreview.com/biomedicine/21196/page2/
Artificial Pancreas Could Revolutionize Treatment Of Type 1 Diabetes
The algorithim, also known as the "smart program" proved to be exceedingly complicated. Researchers in the US and Italy were trying to create a simple program which would be able to pick up the host blood glucose level, and then somehow go into the database and pick out the exact dosage of insulin which would suit the numbers. Basically, these medical engineers needed to devise a system which could compute on a human- like level.
After countless months in development, the FDA stepped in. While developing the silico computer simulation experiments, the FDA granted the researchers approval to test the novel artifical pancreas in humans before the animal trials. This cut the overall development process from several years to six months.
The fact that the FDA eliminated in vivo trials says alot about the artifical pancreas in a biotechnological light. When new technologies and drugs are expedited like this, it signifies the importance and necesity of either. Cancer drugs are often expedited during development because they are extremely needed for cancer patients to maintain health, just like this artificial pancreas; it is absolutely mandatory in regulating Type 1 Diabetes.
With the devotion of research teams around the world, and the involvement of the FDA, I think the artificial pancreas will soon prove to be the best way to treat Type 1 Diabetes. I think in a few years, the artificial pancreas will be less expensive because so many people will need/ want them.
http://www.medicalnewstoday.com/articles/126827.php
Fully Closed
The major variable of the experiment was testing blood glucose levels at various points during the day. They also measured A1C levels and the frequency of hypoglycemic events. The results proved that the variation between the fully closed and hybrid loop were minimal (1 to 2 points). The fully closed system did nevertheless prove to be slightly more efficient. This was due to the fact that the insulin pump could administer pre-meal bolus doses which broke down the initial insulin resistance and smoothed out the blood sugar fluctuation.
Glucose levels with FCL control almost never exceeded 300 mg/dl. 85% of all sensor glucose levels were between 70 and 180 mg/dl in the FCL group during the 24-hour study period. During standard open-loop pump therapy, during which time only 58% of sensor glucose values were between 70 and 180 mg/dl. These subjects were already in excellent control, with mean A1C level of only 7.1%.
Hypoglycemia is a main concern as the best blood sugar control comes with the price of potential hypoglycemia. Hypoglycemia occurred only at nighttime in the experiment. Two patients of the Hybrid control group suffered hypoglycemic events in which blood glucose levels reached 57 (mg/dl). The fully closed loop control group patient reached 51 (mg/dl). These episodes were easily fixed with 15g of carbohydrates.
http://care.diabetesjournals.org/content/31/5/934.full#sec-13
"Automated 'Artificial Pancreas' Controls Blood Glucose Levels In Diabetes Patients For First Time
http://www.medicalnewstoday.com/articles/153002.php
Friday, November 13, 2009
"Closing the loop: Artificial pancreas may be just a few years away"
http://www.endocrinetoday.com/view.aspx?rid=43543
